What’s solid-state batteries?
Solid batteries are regarded as an important development for the future. Their particular advantage is that solid-state batteries do not contain any liquids that could leak or catch fire. For this reason, they are significantly safer, more reliable and more durable than current lithium-ion batteries with liquid electrolyte. At the same time, solid-state batteries have the potential to store more energy in the same space with less weight.
ADVANTAGEs
Solid-state batteries offer the potential for:
- High Energy (higher voltage cathodes possible)
- High Power (large discharge rates possible)
- Low Mass (less inert material)
- High cycle number (10x cycle life of typical Liquid)
MISSION
The aim was to expand our concept for a solid-state battery in such a way that a stable operation with a lithium anode is possible.
Our main research interest at developing fundamental knowledge through experiment and modeling enabling new solid state materials and processes that improve Li-ion battery performance, cost, and reliability.
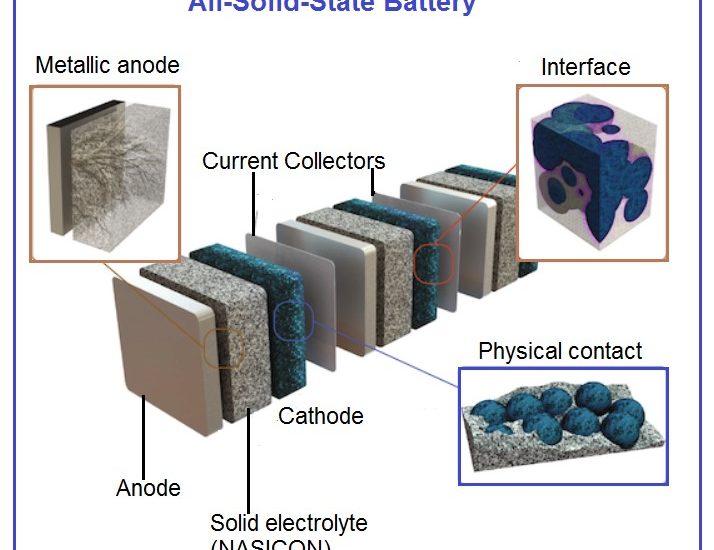
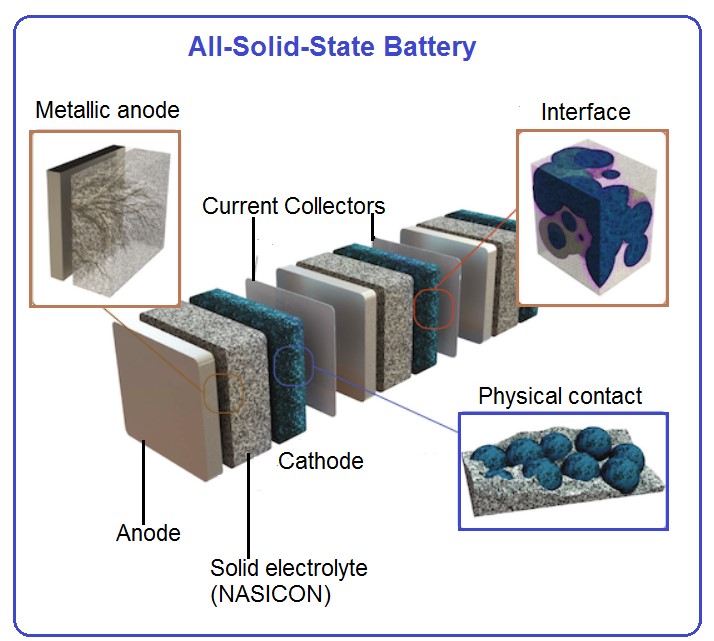
Our lab was fully equipped for solid electrolyte development and testing. State-of-the-art facilities include synthesis lab with fume hood, rotary evaporator, temperature-dependent viscosity, conductivity and density analysis, FTIR spectrometers, glove-boxes, facilities to assemble coin cells, Swagelok cells as well as small pouch cells, electrochemical characterization including battery cyclers is also available. We are currently working on the high lithium ion conducting solid electrolytes based on NASICON materials (see references below)
New promising NASICON material as solid electrolyte for Li-ion batteries
High conductivity values found in the Li1+xTi2–xScx(PO4)3 series open new perspectives for preparing NASICON-based electrolytes for all-solid-state lithium batteries. At present, electrical properties of Li1.2Ti1.8Sc0.2(PO4)3 make this material one of the best reported ion conductors at room temperature (σb ≈ 2.5×10–3 S.cm–1), displaying one of the lowest activation energies reported in NASICON compounds (0.25 eV) (see reference below).
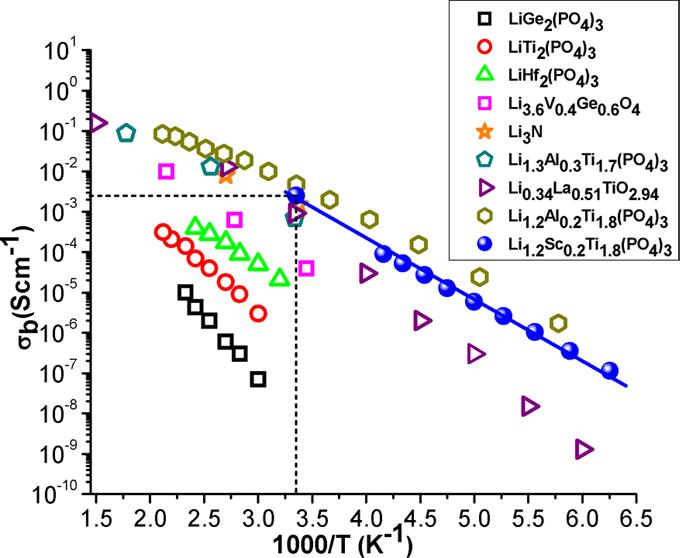
7 orders of magnitude difference in ionic and electronic conductivity
Relevant references
Cation Miscibility and Lithium Mobility in NASICON Li1+xTi2-xScx(PO4)3 (0 ≤ x ≤ 0.5) Series: A Combined NMR and Impedance Study.
Radhouene Kahlaoui, Kamel Arbi, Isabel Sobrados, Ricardo Jimenez, Jesus Sanz and Riadh Ternane, Inorganic Chemistry (2017), 56, 1216-1224. DOI: 10.1021/acs.inorgchem.6b02274. https://pubs.acs.org/doi/abs/10.1021/acs.inorgchem.6b02274